(Total) Recall: Chronicle of an announced war against nitrosamines
When the risk of cancer linked to anti-smoking drugs outweighs the risk linked to smoking itself, it leads to a recall of all Chantix products from Pfizer between July and September 2021: a distribution halting was announced in June 2021, with no less than 154 batches (all concentrations overall) removed from the market and a class action was initiated in New York for “the sale of misbranded drug”.
Beware of the sleeping R1N(-R2)-N=O
Any medication induces a certain number of side effects, with greater or lesser significant probabilities. While reading the paracetamol precautions of use, the benefits/risk balance is easy to determine; however, for other products it can give you a serious headache as it is quite hard to assess. And we are not even talking about the hidden defects to which some drugs are exposed: side effects that are not listed because they were unknown at the time of marketing.
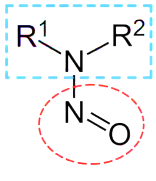 Nitrosamines are a combination of an amine group and a nitroso group. They usually come from a chemical reaction between a secondary amine (to a lesser extent a tertiary amine) and nitrosation agents like nitrites. |
One of those hidden effects are the nitrosamines, a family of mutagenic and carcinogenic impurities, untraced until recently in drugs destined to humans. Their appearance is directly linked to the manufacturing process, to the chemical structure of the molecule, or even to the packaging and storage conditions. Some active ingredients are more prone to this type of contamination: Sartans (used to treat hypertension), Ranitidine (used as an antiacid), Metformine (used to treat diabetes), Rifampin (used as an antibiotic), Varenicline (used in anti-smoking treatments).
Do not believe that the problem raised here is isolated. Nitrosamines and/or their precursors are found in meat, alcoholic beverages, cosmetics, or cigarette smoke. These compounds can also be formed endogenously (in our organism): nitrosamines can be made from a reaction between the stomach/mouth acid and precursors (like nitrites) contained in food. It has been discovered more recently that the disinfection of tap water using dichloramine can lead to traces of nitrosamines.
Do not believe that the problem raised here is isolated. Nitrosamines and/or their precursors are found in meat, alcoholic beverages, cosmetics, or cigarette smoke. These compounds can also be formed endogenously (in our organism): nitrosamines can be made from a reaction between the stomach/mouth acid and precursors (like nitrites) contained in food. It has been discovered more recently that the disinfection of tap water using dichloramine can lead to traces of nitrosamines.
Factors inducing risks of contamination in a pharmaceutical process:
|
Continuity error between food and pharmaceutical industries.
The problem is well known in the food industry (the link between nitrites contained in deli meat and cancerous nitrosamines was already made in 1975), it is however much more recent for drugs destined to humans.
As a matter of fact, it was in 2018 that the Nitrosamines bubble burst, when the FDA (Food and Drug Administration) and EMA (European Medical Agency) announced the recall of generic anti-hypertensives drugs, of which the active substance (Valsartan), manufactured by Zhejiang Huahai, was a cause of contamination. It was revealed that this manufacturer did not respect the good manufacturing practices, by modifying its manufacturing process, gathering all the factors needed for the formation of nitrosamines.
Before that, no guideline concerning nitrosamines was indicated by the FDA or EMA. “Manufacturers are responsible for the comprehension of their process, including the prevention of unacceptable impurities. Manufacturers are also responsible for the development and usage of appropriate methods to detect and limit the unacceptable impurities, including any new impurity that might appear with modifications of the manufacturing process.”. No maximum acceptable daily intake of nitrosamines was specified for its release on the market.
Since then, the hunt of nitrosamines has amplified, the investigation has affected other manufacturers or valsartan, then other drugs. No less than 50 product recalls were done in the US due to nitrosamines since 2019, last in date being the Chantix from Pfizer. In Europe, member countries manage the recall of products. Nevertheless, EMA emitted the recommendation to put on hold all medication containing ranitidine within the European Union, because the nitrosamine content would increase during the product shelf life of the product, due to a stability problem.
New regulatory setting: Lively scenario for the pharmaceutical sector
As of today, both EMA and FDA have published guidance to regulate the presence of nitrosamines in drugs. All manufacturers need to estimate and verify their manufacturing process in search of nitrosamines or precursors, whether their products origin is chemical or biological, although latter presents a small risk according to their manufacturing process.
This is where recall are likely to come in thick and fast! If the nitrosamines were considered as « unstudied chemical that poses a negligible risk of carcinogenicity » until now, with an accepted daily limit of 1.5 mg, this highlight changed everything… The authorities agreed on an acceptable daily exposition to nitrosamines that should not induce more than 1 cancer out of 100 000 after 70 years of exposition. Toxicity studies have brought this limit down to the order of the nanogram (1000 times less than before the Valsartan scandal): from 18 to 96 ng / day depending on the nature of the nitrosamine involved. And any marketed that does not comply with this measure must therefore be subject to a recall.
Product Recall: Big budget feature film
Everyone would agree that this kind of "Recall due to contamination with carcinogenic impurities" note is a tough pill for a company to swallow. First, there are the direct costs linked to the repatriation and destruction of the contaminated doses: the doses will not be sold, which is a first deadweight loss (cost of production, transport, advertising not covered). Instead, distributors will be notified, patients will be informed by the press, units will be repacked, transported, and destroyed. There follows a substantial list of indirect costs to be taken into account: lawsuits, compensation, fall in market value, penalties, loss of certification or insurance, etc. Not to mention the most important long-term risk: the impact on the market reputation and loss of trust of patients. Another sector, same consequences: 6 years after the facts, the Volkswagen emission scandal remains in the memories of consumers! VW has yet to regain consumer confidence or its former glory.
In addition, a product recall can induce an innovation lag as the company focuses on investigating and correcting the defect rather than developing its next technology… Finally, other companies simply have not overcome their product recall. This was the case with Takata, which went bankrupt in 2017, following the introduction of defective airbags, which could explode and project metal shrapnel on the driver and passenger.
End clap for nitrosamines
Faced with these threats, this new legal obligation regarding nitrosamines comes at the right time to protect the patient but also the drug manufacturer. And even if it is true that the evaluation and the reduction of this risk generate a certain cost, this strategy can turn out to be a serious investment because of its long-term payoff!
This investment even goes beyond the manufacturers of the finished product: any manufacturer of raw materials, cleaning products, single use systems and packaging likely to be used in a pharmaceutical process and to leave traces in the finished product has must carry out these analyzes to track down potential components impacting patient safety. The "nitrosamine free" label could become as essential as the "endotoxin free" one. This kind of studies accentuates the customer confidence, based on the transparency, the diligence of the supplier. By taking the initiative, each intermediary in the drug manufacturing process enables safe and effective solutions for the patient to be brought to market.
Finally, nitrosamines are just the latest example. Before them, melamine, endotoxins, prions, among others, modified the health requirements of the marketing of drugs for human use. Others will inevitably follow and reshape the pharmaceutical landscape. And if nitrosamines will soon no longer be among the extras in the pharmaceutical world, their departure is also likely to impact larger players; these drugs for which the producer has identified a nitrosamine risk but does not have the budget to bring them up to date "nitrosamine free". So, there is a whole series of drugs that may no longer be marketed.
The patient may therefore feel cheated, by being withdrawn from an essential drug such as an anti-diabetic or an anti-hypertensive to prevent a risk of 0.001 to 0.01%. Especially when we know that the main source of nitrosamines in our lives is food, where the measures are nowhere near as strict but the pharmaceutical industry has to apply these rules: any additional risk of cancer, however negligible, cannot be tolerated if it can be avoided. So big names may give way to new molecules such as Arnold Schwarzenegger replaced by Colin Farrell. But the next generation is assured, and the show must go on!
Where to start?
Let's start with the most urgent, nitrosamines. If you are taking the nitrosamine train on, know that the strategy chosen by the EMA is in two stages:
#Phase 1: Theoretical risk assessment, including screening of any stage of the manufacturing process, any storage condition, transport of the finished product, but also its degradation during its lifetime, any input material, any product cleaning ... in search of factors inducing the formation of nitrosamines. If such a risk is identified, it must be escalated to the authorities in order to move on to phase 2. For any drug already on the market, the deadline for this assessment was March (drugs of chemical origin) or July (drugs of chemical origin). biological origin) 2021.
#Phase 2: Confirmation of the theoretical risk. The first step is to learn about the mutagenic / carcinogenic nature of the nitrosamine identified in phase 1. If it does not present these characteristics, it can be treated like any other contamination on the basis of the ICH Q3A and ICH Q3B guidelines. Otherwise, analytical tests are set up to quantify the impurity in the finished product. Because it is possible that nitrosamine is formed at the start of the manufacturing process and eliminated during subsequent steps.
Based on these results, a control strategy or modifications to the manufacturing process can be put in place to eliminate the confirmed risk of nitrosamine.
- The EMA has published a page with the different steps and timelines for a nitrosamine risk analysis
- In addition, a new chapter devoted to methods for detecting nitrosamines will appear in edition 10.6 of the European Pharmacopoeia (EP 2.5.42)
- Avertim can help you in this risk analysis, in optimizing your manufacturing processes and in the development, qualification and validation of analytical methods.
Written by

- Arthur C., Solution Senior Manager, Life Sciences industry
- Amandine B., Consultant, Life Sciences industry
