Quality Event Management, CAPA’s and Deviation Management in Life Sciences: Keeping non-conformities in a sustainable state of control.
How confident are you to handle new quality events? Is your QMS robust and sustainable?
- Lack of effective process performance management (e.g. prioritization and visualization)
- Divergent or inadequate level of quality during the deviation investigations and mitigations process
- Recurrence of deviations due to ineffective Corrective and Preventive Actions (CAPA)
We support you on two key topics in this area: quality event handling and the increase of your quality event management processes to keep non-conformities in a sustainable state of control.
1. Quality Event Handling
Our complementary team helps you throughout the whole quality event handling process by working with your manufacturing and quality teams and our team blending in. They implement hands-on solutions, handle your backlog, and can also provide coaching and training to your staff. At Avertim, we follow a robust approach to handle quality events:
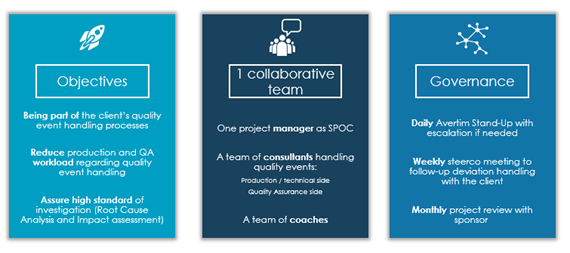
Figure 1 Our Approach to handle your quality events
2. Process Performance Improvment
Handling events is key. But preventing them from happening in the first place is better! In addition to handling quality events, we have developed a proven methodology based on business process management and lean methodologies to ensure your quality processes are robust and efficient on the long run. This is based on several years of experimentation and various successful client cases. Avertim can optimize your quality system thanks to:
- Processefficiency improvement
- Application of a risk-based approach
- Performance visualization
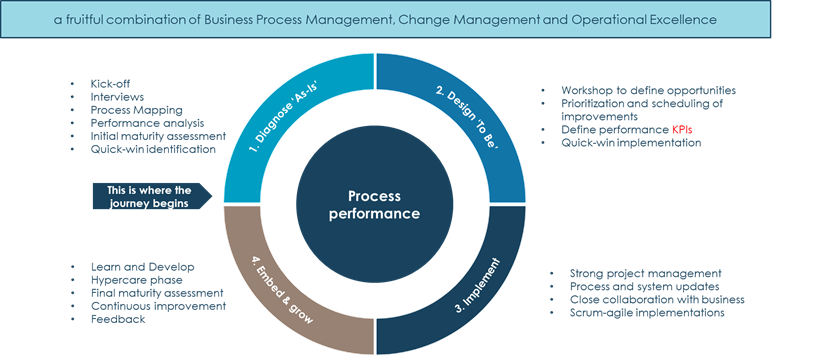
Figure 2 Our approach to boost process performance
In 2020, we delivered the following results:
- Quality Events Handling: 10 service contracts for vaccines manufacturers and CDMO involving 35 consultants and 971 deviations.
- Process performance improvement: 1 service contract to improve the management of quality events increasing on-time closure, a 60% decrease in deviation lead time and closed of 500+ backlog complaints.
Want to discover in more detail our Quality Event Management service?
Please contact us: lifesciences@avertim.com
