166 vaccines in the race against COVID-19 – Germany among the frontrunners
Driven by the impact of the current COVID-19 pandemic on the global healthcare and economy, researchers as well as companies across the world accelerate development of vaccines using different vaccine technology platforms . The WHO tracks the global research efforts against COVID-19 and continuously reports on their progress. Read more about the different vaccines technologies in our last article
As of August 2020, there are 166 vaccine candidates against COVID-19 in different stages of pre-clinical and clinical development.1 The majority of vaccines in development are protein-based, followed by viral-vectors and nucleic acids vaccines, as shown in figure 1

Overview of the most advanced COVID-19 vaccine candidates
27 COVID-19 vaccine candidates worldwide are now in clinical evaluation,while six of them are in phase 3 testing.1 In Germany the promising candidate vaccine BNT162b2 of BioNTech and its partner Pfizer is in clinical phase 3 and more than half of the around 30000 participants have been enrolled.2,3 At the same time, CureVac’s vaccine candidate CVnCoV is in a phase 1 clinical trial and its phase 2 trial is already approved.4,5
The late-stage testing for mRNA 1273 vaccine of Moderna and its cooperation partner NIAID started end of July 2020 and will end in October 2022 with about 30000 participants.6 ChAdOx1-S, a non-replicating viral-vector based candidate of University of Oxford and AstraZeneca is currently in late-stage clinical trial, which will end in October 2022.7,8
Three additional candidate vaccines are being developed by Sinovac, Wuhan institute and Beijing institute. The Ad26.COV2.S vector-based vaccine developed by Janssen Pharmaceutical Companies of Johnson & Johnson is currently in Phase 1/2 clinical testing and Phase 3 with approx. 60000 volunteers is expected to begin in September 2020.9,10Sanofi and GSK intend to kick off a phase 1/2 trial for their candidate vaccine(s) in September 2020, followed by a phase 3 efficacy study by the end of 2020.11
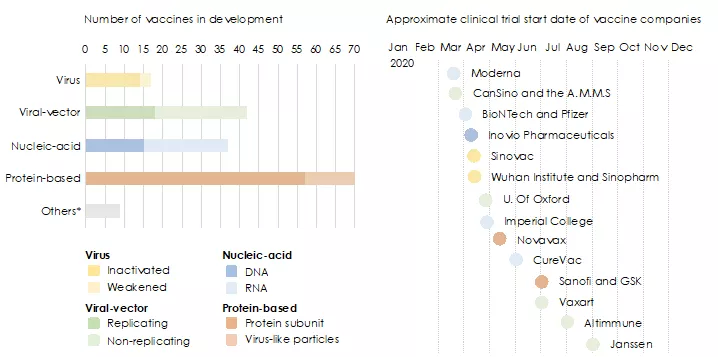
Figure 1: Global activities in vaccine development. *Other efforts include testing whether existing vaccines against poliovirus or tuberculosis could help to fight COVID-19. 1,12,13
Global deals for the development and production have already kicked off
BioNTech and Pfizer announced an agreement with the government of Canada to supply their vaccines BNT162b1 in Phase 2 and BNT162b2 in Phase 3.14 They also made a deal with Japan to provide 120 million doses of BNT162b2. CureVac and Tesla Inc. are cooperating to produce RNA vaccines and a Phase I clinic trial of mRNA vaccine candidate CVnCoV was launched in June 2020.4 Furthermore, the U.S. government has committed $1.95 billion for 100 million doses of the BioNTech/Pfizer vaccine. Should the clinical trials yield positive results, the companies aim to submit a request for Biologics License Application (BLA) by October 2020.15 On that timeline, 100 million doses will be produced worldwide by year's end, and around 1.3 billion doses by the end of 2021. Another supply deal was already signed to provide the U.K. with 30 million vaccine doses over the next two years.16
According to Fierce Pharma, Sanofi and GSK secured up to $2.1 billion from the U.S. government to speed up clinical development and manufacturing of their adjuvanted, recombinant COVID-19 vaccine candidate.11 In addition, they closed a supply deal with the U.K. government to provide up to 60 million doses of their working vaccine candidate.17Novavax made a $1.6 billion deal with the U.S. government for its adjuvanted nanoparticle vaccine candidate to support late-stage vaccine testing and manufacturing.18 In addition, Moderna obtained $2.48 billion from the U.S government for the development and delivery of 100 million doses of its mRNA vaccine candidate.19
You want more information about Avertim services in the Life Sciences & vaccines area?
Visit our page https://www.avertim.com/en/life-sciences-chemicals or Contact us
In the next article we will discuss about the challenges in COVID-19 vaccine development versus traditional vaccines.
References
- WHO. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-can….
- Taylor, N. P. Pfizer, BioNTech share clinical data linking favored COVID-19 vaccine to improved tolerability. https://www.fiercebiotech.com/biotech/pfizer-biontech-share-clinical-da….
- Pfizer says COVID-19 vaccine trial more than 50 percent enrolled. https://www.reuters.com/article/us-health-coronavirus-pfizer-vaccine/pf….
- A Study to Evaluate the Safety, Reactogenicity and Immunogenicity of Vaccine CVnCoV in Healthy Adults. https://clinicaltrials.gov/ct2/show/NCT04449276?term=vaccine&cond=covid….
- A Dose-Confirmation Study to Evaluate the Safety, Reactogenicity and Immunogenicity of Vaccine CVnCoV in Healthy Adults. https://clinicaltrials.gov/ct2/show/NCT04515147?term=vaccine&cond=covid….
- A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19. https://clinicaltrials.gov/ct2/show/NCT04470427?term=vaccine&cond=covid….
- Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults. https://clinicaltrials.gov/ct2/show/NCT04516746?term=astrazeneca&cond=c….
- A phase III study to investigate a vaccine against COVID-19. http://www.isrctn.com/ISRCTN89951424.
- Single Dose of Johnson & Johnson COVID-19 Vaccine Candidate Demonstrates Robust Protection in Pre-clinical Studies. https://www.prnewswire.com/news-releases/single-dose-of-johnson--johnso….
- Johnson & Johnson gears up for 60,000-person COVID-19 vaccine trial, the industry’s biggest yet. https://www.fiercepharma.com/vaccines/johnson-johnson-gears-up-for-60-0….
- Liu, A. Top vaccine players Sanofi, GSK win $2.1B Warp Speed funding for COVID-19 shot. https://www.fiercepharma.com/pharma/sanofi-gsk-win-hefty-2-1b-operation….
- Callaway, E. The race for Coronavirus vaccines. Nature 580, 576–577 (2020).
- Thompson, S. A. How Long Will a Vaccine Really Take? https://www.nytimes.com/interactive/2020/04/30/opinion/coronavirus-covi….
- Pfizer and BioNTech to Supply Canada with their BNT162 mRNA- Based Vaccine Candidate. https://www.newswire.ca/news-releases/pfizer-and-biontech-to-supply-can….
- Vaccine Product Approval Process. https://www.fda.gov/vaccines-blood-biologics/development-approval-proce….
- Kansteiner, F. Pfizer, BioNTech keep COVID vaccine deals rolling with 120M-dose Japan pact. https://www.fiercepharma.com/vaccines/pfizer-and-biontech-keep-supply-d….
- Kansteiner, F. Sanofi, GlaxoSmithKline pledge 60M doses of their recombinant COVID-19 shot to U.K. https://www.fiercepharma.com/manufacturing/u-k-shores-up-60-million-mor….
- Sagonowsky, E. Novavax inks $1.6B Warp Speed deal to fund COVID vaccine’s phase 3 testing, manufacturing. https://www.fiercepharma.com/pharma/novavax-inks-1-6b-warp-speed-deal-t….
- After nearly $1B in research funding, Moderna takes $1.5B coronavirus vaccine order from U.S. https://www.fiercepharma.com/pharma/after-nearly-1b-research-funding-mo….
